Contact

+86-21-57526862
Tel:+86-21-57526862
Email:
sales@eugenebio.com
Address:No. 1666 Xinyang road, Fengxian district, Shanghai, China
-
Dengue IgG/IgM
Tel:+86-21-57526862

Packing specification:25T
Intended use:
The EUGENE Dengue IgG / IgM Rapid Test is a lateral flow immunochromatographic assay for the rapid qualitative detection and differentiation of IgG and IgM antibodies to dengue virus in human whole blood, serum or plasma. It is intended for professional use to aid in the presumptive diagnosis for primary and secondary dengue infection. This test provides only a preliminary test result. Therefore, isolation of virus, antigen detection in fixed tissues, RT-PCR and serological test like haemagglutination-inhibition test, more specific alternative diagnosis method must be used in order to obtain a confirmation of dengue virus infection.
TEST PROCEDURE :
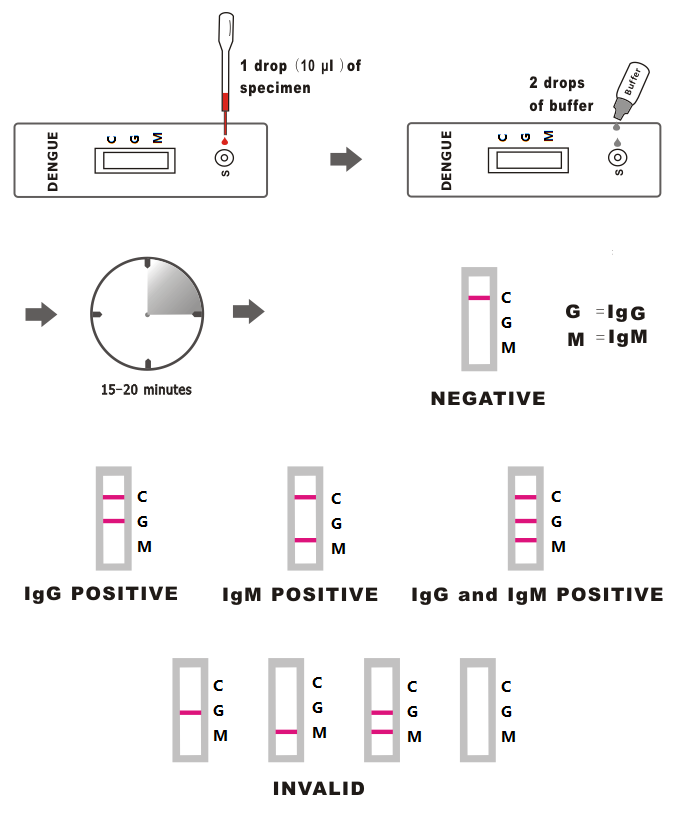
1.Remove the test device from the foil pouch and place it on a clean and flat surface. Be sure to label the device with specimen’s ID number.
2.Use the provided dropper or a micropipette to add 1drop (10ul) of serum, plasma or whole blood specimen into the sample well (S). Then add 2 drops of assay buffer (about 80ul) to the sample well. And start the timer.
3.Interpret test results at 15-20 minutes. Don’t read result after 20 minutes.
Possible setting for the test: Home, workplace, physician’s office or clinical labs
Storage conditions and expiration date:
1. The original package shall be stored at 4℃ ~ 30℃, and shall not be frozen. The validity period is 24 months from the date of verification and qualification.
2. The test paper should be used within 1 hour after the original aluminum foil bag is opened.
 中文版
中文版 English
English